Product Center
Vitamin B6 1ml
Please read the instructions carefully and use under the guidance of a physician
Generic name: Vitamin B6 injection
English name: Vitamin B6 Injection
Chinese Pinyin: Weishengsu B6 Zhusheye
【Ingredients】
Chemical name: 6-methyl-5-hydroxy-3,4-pyridine dimethanol hydrochloride.
Chemical Structure:
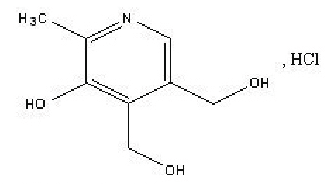
Molecular weight: 205.64
The excipients are: sodium chloride, hydroxypropyl beta-cyclodextrin, sodium hydroxide, and water for injection.
【Indications】
1. It is suitable for the prevention and treatment of vitamin B6 deficiency and isoniazid poisoning; it can also be used for pregnancy, radiation sickness and vomiting and seborrheic dermatitis caused by anticancer drugs.
2. Total parenteral nutrition and supplementation of vitamin B6 during malnutrition and progressive weight loss due to insufficient intake.
3. The following conditions increase the need for vitamin B6: pregnancy and lactation, hyperthyroidism, burns, long-term chronic infections, fever, congenital metabolic disorders (cystathionuria, hyperoxalemia, homocystine Urine disease, xanthineuria), congestive heart failure, long-term hemodialysis, malabsorption syndrome with hepatobiliary diseases (such as alcoholism with cirrhosis), intestinal diseases (celiac disease, tropical stomatitis, limited Enteritis, persistent diarrhea), after gastrectomy.
4. Neonatal hereditary vitamin B6 dependence syndrome.
【Specifications】1ml:50mg
【Dosage】
Subcutaneous injection, intramuscular or intravenous injection, 50 mg to 100 mg once, once a day. When used for the detoxification of cycloserine poisoning, 300mg or more per day. When used for isoniazid poisoning and detoxification, give 1g of vitamin B6 intravenously for every 1g of isoniazid.
【Adverse reactions】
Vitamin B6 produces almost no toxicity when kidney function is normal. Rare allergic reactions. If 200mg is applied daily for more than 30 days, it can cause dependency syndrome.
[Taboo] Not clear yet.
【Precautions】
1. Vitamin B6 has not been proved to be effective for the following conditions, such as acne and other skin diseases, alcoholism, asthma, kidney stones, psychosis, migraine, premenstrual tension, stimulation of milk secretion, and loss of appetite. High-dose vitamin B6 should not be used to treat diseases that have not been proven effective.
2. Vitamin B6 affects the efficacy of levodopa in the treatment of Parkinson's disease, but has no effect on the efficacy of carbidopa.
3. Interference with diagnosis: the urobilinogen test is false positive.
【Medication for pregnant women and lactating women】Pregnant women receiving large amounts of vitamin B6 can cause vitamin B6 dependence syndrome in newborns. The normal intake of lactating mothers has no adverse effects on the baby.
【Children's Medication】It is not clear yet.
[Medication for the elderly] It is not clear yet.
【medicine interactions】
1. Chloramphenicol, cycloserine, ethionamide, hydralazine hydrochloride, immunosuppressive agents including adrenal cortex hormones, cyclophosphamide, cyclosporine, isoniazid, penicillamine and other drugs can antagonize vitamin B6 or Increasing the excretion of vitamin B6 through the kidneys can cause anemia or peripheral neuritis.
2. The amount of vitamin B6 should be increased when taking estrogen.
3. Levodopa combined with a small dose of vitamin B6 (5mg per day) can antagonize the anti-tremor effect of levodopa.
[Drug overdose] Daily application of 2-6g for several months can cause severe neurosensory abnormalities, progressive gait instability, foot numbness, and hand inflexibility. It can be relieved after stopping the drug, but it is still weak.
【Pharmacology and Toxicology】
This product is a vitamin medicine. Vitamin B6 is converted into pyridoxal phosphate in red blood cells, which acts as a coenzyme on various metabolic functions of protein, carbohydrates and lipids, and also participates in the conversion of tryptophan into niacin or serotonin.
【Pharmacokinetics】
Vitamin B6 does not bind to plasma proteins, and pyridoxal phosphate can bind to plasma proteins. The t1/2 of vitamin B6 is as long as 15-20 days. Metabolism in the liver. Excreted by the kidneys. Can be discharged through hemodialysis.
[Storage] Shade and keep in airtight.
[Packing] Glass ampoules, 10 pcs/box.
[Validity period] 30 months.
[Executive standard] "Chinese Pharmacopoeia" 2020 edition two
[Approval number] National Medicine Standard H42021520
[Marketing Permit Holder]
Name: Hubei Minkang Pharmaceutical Co., Ltd.
Registered address: No. 50, Xiba Road, Yichang City, Hubei Province
【manufacturer】
Commissioner: Hubei Minkang Pharmaceutical Co., Ltd.
Registered address: No. 50, Xiba Road, Yichang City, Hubei Province
Trustee: Hubei Meilin Pharmaceutical Co., Ltd.
Production address: No. 8, Heping Road, Economic Development Zone, Yunmeng County, Xiaogan City, Hubei Province
Postal Code: 443002
Phone number: (0717) 6272141 (0717) 6276908
Fax number: (0717) 6272162
Web site: http://www.minkang.com.cn
Related Products
Inquiry
Contact information
Online Message
Business Email:hbmlyy@merriclin.cn HR Email:hr@merriclin.cn
Company address:No. 8 Heping Road, Economic Development Zone, Yunmeng County, Xiaogan, Hubei Postal code:432500 This website supports IPV6
Copyright © 2012-2021 Hubei Merriclin Pharmaceutical Co.,Ltd. Powered by www.300.cn
Filing information:鄂公网安备42092300000000号 鄂ICP备20010943号-1 《Internet information service qualification certificate》No.(E) - non operating-2020-0023
Operation information: the website has been running for 00001 days. It is recommended to use the browser based on chromium core to browse the website at a resolution of no less than 1920 * 1280px to obtain good browsing effect.

Merriclin Official Website

Merriclin WeChat Official









 Home
Home About Us
About Us News
News Products
Products R & D Center
R & D Center HR
HR Help Center
Help Center Contact Us
Contact Us