News Center
The list of key monitoring drugs may be adjusted every two years, and 30 varieties will be included
- Categories:Media reports
- Author:
- Origin:Medical Cloud Studio
- Time of issue:2021-08-23
Medical Network, August 23. Following the start of a new round of adjustments to the medical insurance catalogue and repeated news about the adjustment of the basic medicine catalogue, the key monitoring drug catalogue is also about to be adjusted. The catalogue previously included 20 varieties, which may reach 30 after the adjustment. Varieties.
Recently, the industry has issued a letter entitled "The National Health Commission's Medical Administration and Hospital Administration's Letter of Soliciting Opinions on the National Key Monitoring and Reasonable Use of Drugs Catalogue Adjustments (Draft for Solicitation of Comments)". This means that the new version of the key monitoring catalog will be released soon.
On July 1, 2019, the "National Key Monitoring and Rational Use Drug List" was officially released, and 20 varieties were included.
The National Health Commission stated that the fundamental purpose of formulating the national key monitoring and rational use of drugs is to standardize medical behavior and improve the level of rational use of these drugs in the clinic. For the drugs in the catalog, it is required to use the prescribed course of treatment and dosage reasonably under the condition of strictly grasping the medication indications.
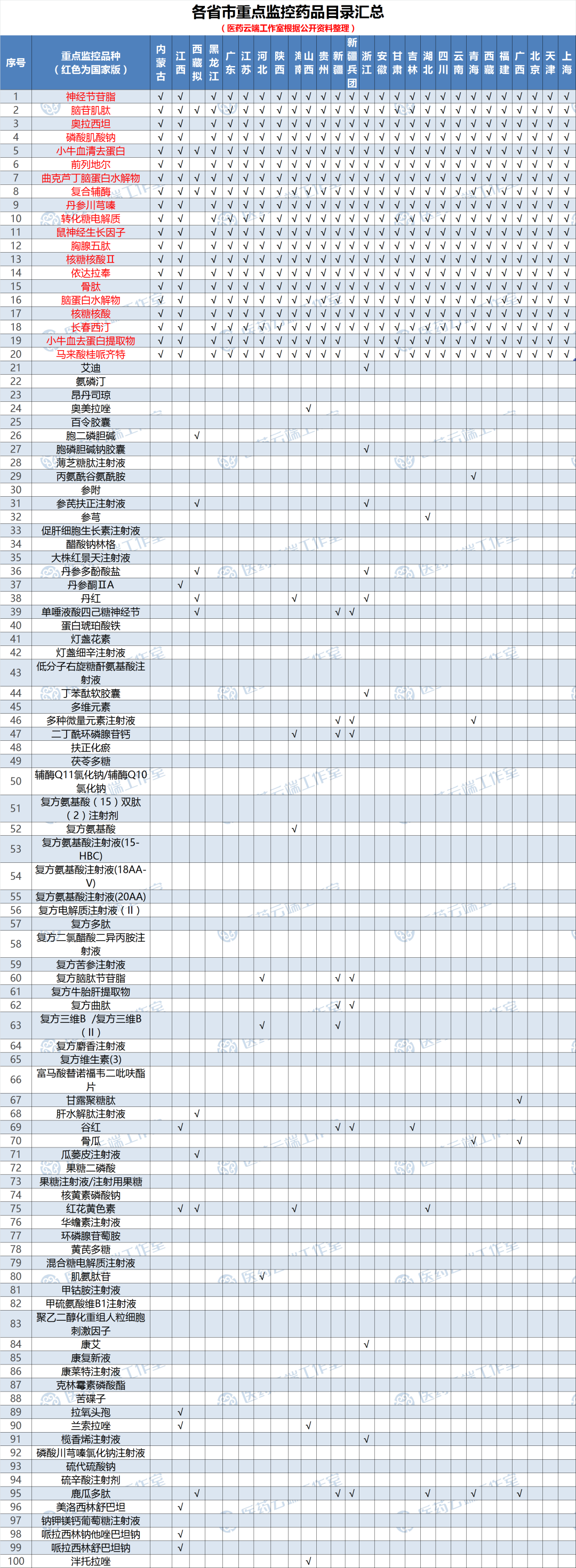
Provinces and cities have announced more than 200 key monitoring drugs, these varieties may have been locked
At present, various provinces and cities have successively issued a list of key monitoring drugs in various regions. According to incomplete statistics, in addition to the national version of 20 varieties, provincial, municipal, and even medical institutions have conducted key monitoring of certain varieties, involving chemical drugs and biological drugs. More than 200 medicines including medicines and Chinese patent medicines.
If there is no accident, the newly added key monitoring drugs will be reported to the final catalogue layer by layer within this range. These varieties may have been locked! Interestingly, some of them have entered the local volume purchase.
As shown in the figure below: All provinces and cities without screenshots due to space limitations
Below, let’s take a look at the key contents of the "Working Procedures for the Adjustment of the National Key Monitoring and Rational Use Drugs List (Draft for Solicitation of Comments)":
Chemical and biological drugs that are clinically unreasonable, expensive, and have a greater impact on the rationality of medication should be included
The drugs included in the catalogue management should be chemical drugs and biological products that have a lot of unreasonable clinical use problems, a significantly higher amount of use, and a greater impact on the rationality of the medication. The focus includes adjuvant drugs, anti-tumor drugs, antibacterial drugs, proton pump inhibitors, glucocorticoids, parenteral nutrition drugs, etc.
The adjustment of the catalog adheres to the principle of "openness and transparency, local recommendation, and dynamic adjustment", and aims to standardize clinical drug use and promote rational drug use.
In principle, the time for updating and adjusting the catalogue should not be less than 2 years, and the number of drug varieties included in catalogue management is generally 30.
The National Pharmaceutical Administration and Pharmacotherapy Committee (hereinafter referred to as the National Pharmaceutical Council) provides professional technical support for the scientific adjustment catalog, and undertakes the compilation, sorting and analysis of recommended materials from various places.
The adjustment of the catalog includes four stages: start-up adjustment, local selection and recommendation, expert summary, and results announcement.
(一) Start adjustment. The National Health Commission issued a notice of catalogue adjustments, clarifying the time schedule, materials to be submitted, work requirements, etc.
(2) Local selection and recommendation. According to the comprehensive factors such as the clinical value of the drug, the current status of clinical irregular use, and the amount of use in general hospitals above the second level, after research and selection by the hospital's pharmaceutical management and pharmacotherapy committee, they will not distinguish the dosage form and use the generic name of the drug according to the degree of recommendation. To the weak ranking, report the information of the top 30 varieties with the strongest recommendation to the provincial health committee.
The Provincial Health Commission assigns corresponding scores to the 30 varieties recommended by each hospital, that is, the first-ranked variety is assigned a value of 30, and the second-ranked variety is assigned a value of 29. This rule decreases and ranks 30th. Bit variety
is assigned 1 point. On the basis of the generic name of the drug, the provincial health commission sums up the assignments of all the varieties submitted by general hospitals above the level of jurisdiction to obtain the total recommended score for each variety, and then sorts them from high to low. , To report the information of the first 30 varieties to the Medical Administration and Hospital Administration of the National Health Commission.
(3) Expert summary. The Medical Administration and Medical Administration of the National Health Commission entrusted the National Pharmaceutical Affairs Commission to conduct a formal review of the materials submitted by various regions, and adopt the same calculation method as that of the provincial health commissions. After adding the value of the drug variety, the calculation results in the top ranking 30 varieties.
(4) Announce the results. The National Health Commission announced the results of catalog adjustments, released a new version of the drug catalog for key monitoring and rational use, and put forward management requirements.
The provincial catalog should add the national catalog variety in time, and the drugs from the original catalog should be recalled, and the locality should continue to monitor for at least 1 year
All provinces should refer to the national catalogue adjustment procedures to form a provincial catalogue and compare it with the national catalogue. If the national catalogue drugs are not included, they should be added to the provincial key monitoring and rational use drug catalogue in a timely manner. The final provincial-level key monitoring and rational use drug catalog should be published in time and reported to the National Health Commission for the record.
For the drugs adjusted out of the original catalogue, the local health administrative department should continue to monitor for at least one year, and grasp the prescription review, usage, usage amount, etc., to promote the continuous improvement of the level of clinical rational use of drugs.
RELATED NEWS
Business Email:hbmlyy@merriclin.cn HR Email:hr@merriclin.cn
Company address:No. 8 Heping Road, Economic Development Zone, Yunmeng County, Xiaogan, Hubei Postal code:432500 This website supports IPV6
Copyright © 2012-2021 Hubei Merriclin Pharmaceutical Co.,Ltd. Powered by www.300.cn
Filing information:鄂公网安备42092300000000号 鄂ICP备20010943号-1 《Internet information service qualification certificate》No.(E) - non operating-2020-0023
Operation information: the website has been running for 00001 days. It is recommended to use the browser based on chromium core to browse the website at a resolution of no less than 1920 * 1280px to obtain good browsing effect.

Merriclin Official Website

Merriclin WeChat Official


 Home
Home About Us
About Us News
News Products
Products R & D Center
R & D Center HR
HR Help Center
Help Center Contact Us
Contact Us